Plachta Lab
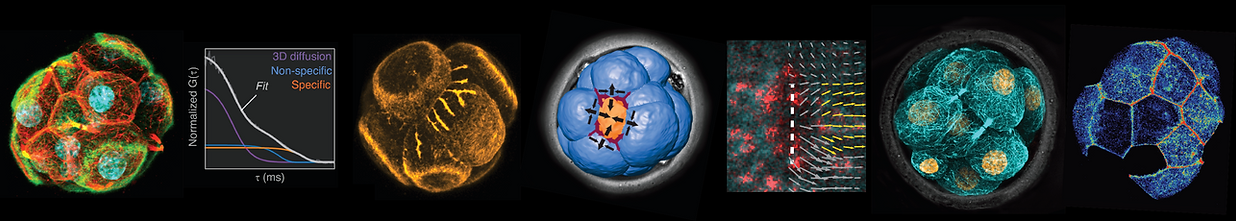
Our lab has pioneered the use of live-imaging to uncover key mechanisms and new forms of cellular organization critical for early development.
The findings help to transform the field of preimplantation development by showing how diverse processes occurring at the level of transcription dynamics, cytoskeletal organization, and the action of mechanical forces are integrated in real time to ensure proper embryo development.

The preimplantation embryo offers an excellent in vivo system. This mouse embryo was microinjected with mRNA to label the chromatin (green) and cell membranes (red) across development.
Solving how transcription factors control cell fate


Transcription factors (TFs) are essential regulators of cell fate, but their DNA–binding dynamics in vivo had remained unknown. We developed methods to reveal the DNA–binding properties of TFs in live embryos.
Combining fluorescence correlation spectroscopy (FCS) with photo-activation, we performed the first quantification of DNA–binding dynamics in live embryos. This unconvered differences in the dynamics of Oct4 and Sox2 at early stages of development predicting the specification of the first differentiated mammalian cell lineages, which comprise the pluripotent inner mass that forms the foetus, and the trophectoderm that forms the placenta.
White et al. Long-Lived Binding of Sox2 to DNA Predicts Cell Fate in the Four-Cell Mouse Embryo
Cell (2016)
Kaur et al. Probing transcription factor diffusion dynamics in the living mammalian embryo with photoactivatable fluorescence correlation spectroscopy.
Nat Comms (2013)
Plachta et al. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo.
Nat Cell Biol (2011)


Revealing how cells adopt specific positions and fate is key to understanding embryo morphogenesis. We combined live-imaging with computer segmentation to reveal how cells physically segregate to form the pluripotent inner mass in the living mouse embryo. Unlike models based on oriented cell divisions, we showed that the pluripotent mass forms by a morphogenetic process known as apical constriction. We revealed how anisotropies in cortical tension (a force generated by contractility of the actomyosin cell cortex) drives this morphogenetic process.
Samarage et al. Cortical tension allocates the first inner cells of the mammalian embryo.
Dev Cell (2015)
We then showed how cells sense their position to adopt correct lineage fate (pluripotent inside and trophectoderm outside). Interestingly, we showed that the nuclear lamina acts as a mechanosensor which enables coupling the forces driving inner-outer cell segregation to the transcription factors that specify pluripotent versus trophectoderm fate.
Skory*, Moverley*, et al. The nuclear lamina couples mechanical forces to cell fate in the preimplantation embryo via actin organization.
Nat Comms (2023)


Discovering how mechanical forces form the first lineages of the embryo
Finding new forms of microtubule organization
The microtubule cytoskeleton of most animal cells is organized by centrosomes. However, the early mouse embryo lacks centrosomes and it remained debated how microtubules are organized during early development.
We discovered two entirely new structures assembled by microtubules in the embryo. We first found that when cells divide, they do not abscise their cytokinetic bridge. Instead, they maintain their connecting bridge throughout interphase and transform it into a non-centrosomal microtubule organizing center (MTOC). This MTOC maintains sister cells together and directs the transport of key proteins to the cell membrane.
Zenker et al. A microtubule-organizing center directing intracellular transport in the early mouse embryo.
Science (2017)

We also found that a subset of cells in the embryo forms a highly atypical mitotic spindle. Despite lacking centrosomes, this spindle has a large astral microtubule array on its apical side. Importantly, this 'monoastral' spindle causes cells to divide with asymmetric patterns, with daughter cells having differences in volume, polarity, and ability to undergo internalization.
Pomp et al. A monoastral mitotic spindle determining lineage fate and position in the mouse embryo. Nat Cell Biol (2022)


New actin structures driving embryo morphogenesis
Embryo compaction is critical for proper development. We found that the cells of the early embryo form long protrusions (or filopodia) containing E-cadherin and actin, which they use to draw their neighboring cells closer to achieve compaction.
Although filopodia are critical for wound healing, signalling, and cancer, current systems to study cell protrusions in mammalian cells are restricted to culture conditions. Thus, we now study the filopodia of the early embryo to expose how mammalian cells regulate these protrusions in vivo.
Fierro-Gonzalezet al. Cadherin-dependent filopodia control preimplantation embryo compaction.
Nat Cell Biol (2013)

The transformation from morula to blastocyst is another defining event of mammalian development. During this transition, the embryo must establish the first permeability barrier to enable expansion of the blastocyst cavity, yet the mechanism triggering this sealing was unknown.
We found that the outer cells of the embryo form a prominent actin ring at their apical cortex. Unlike other ring structures which are enriched in myosin II and contractile, these rings have low myosin II levels and instead, they expand over the entire surface of the embryo. When the rings of neighboring cells contact each other at cell-cell junctions, they undergo a 'zippering' process that triggers formation of tight junctions and seals the embryo for blastocyst formation.
Zenker J et al. Expanding actin rings zipper the mouse embryo for blastocyst formation.
Cell (2018)
Keratins as asymmetrically-inherited fate determinants

Unlike actin and microtubules, the role of intermediate filaments in early development has remained unknown. Keratins are the main intermediate filaments in the early embryo and have always been thought as a trophectoderm marker. We found that some cells form a keratin network before others. Moreover, live-imaging revealed that keratins are asymmetrically inherited during cell division by the future outer cell. Keratin inheritance triggers apical polarization, critical for trophectoderm specification.


But importantly, keratins also stabilize the cortex promoting formation of the monoastral mitotic spindles. Thus, keratin are not only inherited factors that help cells to adopt trophectoderm identity, they also bias division patterns via the monoastral spindle. Finally, we found that keratin expression is differentially regulated by epigenetic differences between cells at early stages. This provides a mechanistic link to understand how early heterogeneities in the embryo start to bias cell lineage fates.

Keratins stabilize cortex
Stable cortex promotes monoastral spindle
Monoastral spindle drives asymmetric division patterns and lineage segregation
Pomp et al. A monoastral mitotic spindle determining lineage fate and position in the mouse embryo.
Nat Cell Biol (2022)
Lim et al. Keratins are asymmetrically inherited fate determinants in the mammalian embryo.
Nature (2021)